Soil acidity – part II: correcting the problem
2007-06-01
In part I of this two part series, the chemistry of
soil acidity and the reasons for restricted crop plant were discussed. It
was explained that pH (KCl) was a good indication of the activity of H+ ions
in the soil but that H+ is actually not the gremlin.
In hierdie artikel:
- Liming and neutralisation of soil
acidity
- Lime requirement indicators
- Soil pH.
- Basic cation saturation ratios
- Acid saturation percentage
- Conclusion
- References
- Graphics
The cause of poor crop growth is due to the associated chemistry that
occurs at low pH, i.e. toxic levels of soluble aluminium plus phosphate and
molybdate fixation. In fact, the measurement of soil pH can be likened to
taking the temperature of a patient: it indicates that something is wrong
and remedial action must be taken.
Liming and neutralisation of soil acidity
Where a soil has become acidic and Al3+ and H+ are dominant ions on the
cation exchange surface, an agricultural lime such as calcitic lime (CaCO3)
needs to be added. The reaction of CaCO3 in acidic soil could probably
proceed as follows:
2CaCO3 + 2H2O = 2Ca2+ + 2HCO3- + 2OH-
The speed of this reaction and the rate that the CaCO3 dissolves is
determined by the rate OH- and HCO3- is removed from the solution. The Ca2+
will displace the H+ and aluminium ions from the clay surface into the soil
solution and the following neutralisation reactions will occur:
H+ + OH- = H2O
H+ + HCO3- = H2O +CO2
The aluminium ions in the solution will undergo the following reactions:
Al(OH)2+ + OH- = Al(OH)2+
Al(OH)2+ + OH- = Al(OH)3
As the neutralisation reaction proceeds, the toxic aluminium is precipitated
as Al-hydroxy-polymers and removed from the solution. With time, these
polymers will slowly crystallise to gibbsite, an insoluble form of aluminium.
The neutralisation of the exchangeable forms of Al and H is a quick process.
It must be noted that the Ca2+ or Mg2+ ions are not responsible for the
neutralisation of H+ and Al3+, it is the OH- and HCO3- ions. A compound such
as gypsum (CaSO4) can not neutralise acidity. It can only act as a source of
calcium and sulphate.
Lime requirement indicators
Unfortunately, there is still some misunderstanding as to which is the best
indicator for lime requirement. Sometimes the acidity component is
mistakenly disregarded in favour of the use of the basic cation saturation
ratio (BCSR). Therefore, it is appropriate to briefly consider the
approaches.
Soil pH
Dr Mart Farina of the Fertiliser Society of South Africa, has presented a
very conclusive data set indicating the relationship between pH and maize
yields recorded in SA, which is presented in Figure 1. The data
clearly shows that there is no correlation between maize yield and soil pH.
When one considers that pH measures H+ and that H+ is not toxic to plants in
the pH ranges encountered in most soils, this feature is not surprising.
Basic cation saturation ratios
This concept originates from the “Albrecht approach”, where the exchangeable
Ca and Mg should ideally constitute approximately 65% to 75% and 10% to 15%,
respectively, of the soil’s cation exchange capacity (CEC). It is also
claimed that the ratio of Ca to Mg should be in the range of 4,3 to 7,5 when
both cations are expressed on an equivalent weight concentration basis. Soil
and yield data from 269 data points of the Ten Ton Maize Club are presented
in Figure 2. This data does not support the existence of an optimum
Ca/Mg ratio. Considering the causes of poor growth in acid soils and the
chemistry of the neutralisation process, it is not surprising that there is
no relationship between maize yield and Ca/Mg ratio.
Acid saturation percentage
Acid saturation percentage is defined as the total acid cations in
chemically equivalent terms expressed as a percentage of the total cations,
i.e. the basic cations plus the acid component. This provides an index of
the Al activity levels.
Figure 3 shows the relationship between yield and acid saturation
percentage. It is seen that as long as the acid saturation is less than 22%,
yields are not reduced. When acid saturation exceeds 22% then the
detrimental effects of Al toxicity become limiting. In the December 2002
issue of Grain SA, dr HJ Venter reported on twelve field trials and
confirmed that correcting soil acidity to 20% acid saturation had positive
results, but additional Ca and Mg applications had no further improvements.
Conclusion
When the chemistry associated with acid soils and neutralisation as already
outlined is considered, it is understandable that acid saturation percentage
is a good indicator of liming requirement, since it aims at eliminating the
main culprit of poor growth with acid soils, i.e. toxic aluminium, from the
soil.
References
MPW Farina and P. Channon, 1991, Plant – soil interactions at low pH, page
465 - 473, Kluwer Academic Publications.
PS Fouché and WJ Fölscher, 1975, Fert.Soc. of South Africa Journal 2: page
67 - 72.
HJ Venter, 2002, CA- en MG-tekorte wanneer geen kalk nodig is nie. SA Graan:
Des 2002, page 33.
Dr Neil du Sautoy is a senior soil scientist with Senwes Agricultural
Services. If you require further information about soil acidity and liming,
please phone him on (018) 464-7391 or 082 419 0949. You can also speak to
Bernard Muller on 083 458 1293.
Graphics
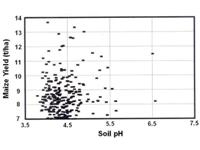
The relationship between maize yield (t/ha) and soil pH (MPW Farina,
Fertilizer Society of SA). |

The relationship between the exchangeable calcium to magnesium ratio
and maize grain yield (MPW Farina, Fertiliser Society of SA). |
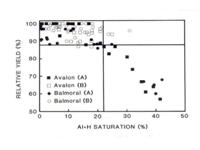
The relationship between acid saturation percentage and maize yield
on soils with clay contents of 10% - 14% (Avalon) and 74% - 76% (Balmoral)
(MPW Farina, Fertiliser Society of SA). |
|


