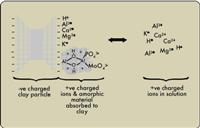
Diagrammatic representation of the nutrient elements and acid components held by the clay and in the soil solution.
What is a soil pH measurement?
Over the years a laboratory technique has been developed that indicates the
acidity of the soil and is referred to as soil pH. It is generally accepted
that the pH(KCl) of a soil is a good indicator of soil acidity. The pH(KCl)
value is when a diluted solution of potassium chloride (KCl) is used in the
analysis, instead of water (H2O) which gives the pH(H2O) value. The pH(KCl)
values are generally one pH unit lower than pH(H2O) values. If a soil has a
pH(KCl) of less than 4,8 it can be regarded as being acidic.
In essence, a soil pH reading is like taking the temperature of a patient.
If a patient has temperature above 37°C it is an indication that he/she has
an infection; the higher the temperature, the more severe the infection.
However, it does not indicate what the infection is and how much medication
is needed. Similarly, if a soil records a pH(KCl) less than 4,5 it is an
indication that there is an acidity problem; but it does not indicate the
amount of lime required.
Interactions between soil components
Within a soil there is a dynamic interaction between the clay, soil solution
and dissolved ions. This interaction is diagrammatically illustrated in
Figure 1. The clay minerals are very small soil particles that have a
negative charge and have a very important role in holding cations such as
calcium (Ca2+), magnesium (Mg2+), potassium (K+) and ammonium (NH4+) in the
soil. These are important plant nutrients that are taken by the plant from
the soil solution. As they are removed by the plant so they can be replaced
in the solution from the soil surface. If the concentration in the solution
is increased (by adding fertiliser/lime), they are absorbed again by the
clay. The process of cations being held on the clay surface is important
because if it did not take place, all the dissolved nutrients would be lost
through leaching as water passed through the profile. This is the reason why
sandy soils are inherently infertile.
In addition to the nutrient cations there are also acid ions. These are the
positively charged hydrogen protons (H+), as well as soluble aluminium ions
such as Al3+, Al(OH) 2+ and
Al(OH) 2+. These entities are in the solution and cause the acidic reactions
that are identified when the soil pH is measured.
In certain soils there are amorphous (shapeless) gels that can be positively
charged. This gel is made up of aluminium and iron atoms linked by oxygen
atoms to form long chains known as polymers. The amorphous component is
effectively not a solution and is thus not reflected when pH is measured.
However, it is not as stable as the crystalline clay material and can bind
or release H+ ions depending on the H+ prevalence in the solution. If there
are lots of H+ ions, they become attached to the amorphous component and
increase its positive charge. However, if the H+ ions are removed from the
solution (e.g. through neutralisation, i.e. liming) the amorphous compounds
give up H+ ions with a reduction in positive charge. This process of taking
up and releasing H+ ions tends to keep the pH stable and is known as
buffering.
Thus a soil with a considerable amount of amorphous material is well
buffered, i.e. the pH does not change easily and once it is acidic, requires
lots of lime to neutralise. Generally sandy soils have negligible amounts of
amorphous material. The amorphous component is prevalent in the highly
weathered clay soils occurring in the high rainfall eastern areas of South
Africa.
The detrimental effects of soil acidity
The important point to note is that the H+ ion per se is not toxic to plants
in the pH ranges encountered in soil. The chemical conditions that are
associated with low pH are the problem. The main gremlin is the soluble
aluminium (Al3+) which is toxic to plants. In addition, as soil acidity
increases (i.e. more H+ and Al3+ ions occur in the soil solution) they
displace the Ca2+, Mg2+, K+ and NH4+ from the clay surface into the
solution. These cations are then flushed from the soil. This is one of the
main problems with acidity, i.e. the basic cations: Ca2+, Mg2+ and K+,
(which are plant nutrients) are lost and H+ and Al3+ dominate the exchange
complex. So besides an excess of toxic aluminium, the plant has to deal with
a shortage of basic nutrients. Calcium has an important function within the
plant in maintaining the functioning of the cell membrane. A cell membrane
low in Ca allows excessive amounts of manganese into the plant and then
manganese toxicity occurs.
In Figure 1 it is seen that the amorphous material also blocks the exchange
sites on the clay. This effectively reduces the storage capacity for basic
nutrient cations. The amorphous material also entraps phosphate (PO43-) and
molybdate (MoO42-) anions. Not only is phosphate availability reduced, but
molybdate, which is vital for phosphate translocation within the plant,
becomes unavailable.
Part II of this series will be published in the next edition of the
Senwester.
References
P.S. Fouche & W.J Fölscher 1975 Fert. Soc. of South Africa Symposium.
M.P.W Farina & P. Channon 1991 Plant-soil interactions at low pH, 465 – 473
Kluwer
Acedemic Publ.
Dr Neil du Sautoy is a senior soil scientist with Senwes Agricultural
Services. Contact him on (018) 464-7391 or 082 419 0949.
 Diagrammatic representation of the nutrient elements and acid components held by the clay and in the soil solution. |